Warehousing
Logistics of pharmaceuticals
Based on our proven track record spanning about 40 years, over 1,000 highly experienced staff provide optimal logistics services for pharmaceuticals.
Outline of services

- DP-Cool GDP-compliant refrigerated delivery service for pharmaceuticals
-
Mitsubishi Logistics offers a refrigerated delivery service for pharmaceuticals throughout Japan. This service includes dedicated and validated vehicles, dock shelters directly connected to refrigerated storage, a temperature monitoring traceability center, and education and training systems.
- Room-temperature GDP transportation
- Utilizing the experience and knowledge gained through the DP-Cool service, we provide comparable services at room temperature throughout Japan.
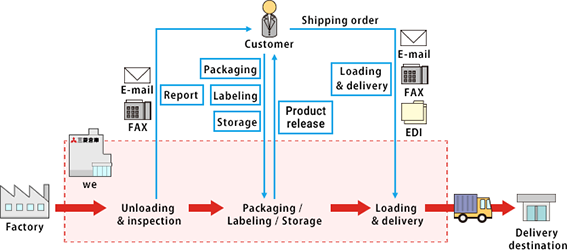
- Pharmaceuticals manufacturing operations (packaging, labeling, and storing)
- We can carry out the process from the acceptance of pre-market products to the product release. By setting up manufacturing sites in the same location as distribution centers, customers can benefit from the reduction of transportation fees and labor costs.
- International transport operations
- We can perform various operations such as the import of pharmaceuticals and active pharmaceutical ingredients (APIs) from overseas suppliers and their export to overseas destinations.
- Order taking operations
- We take orders from the customer's clients via JD-net or by fax on behalf of the customer, and issue a shipping order to our distribution center.
Operations at a pharmaceuticals distribution center
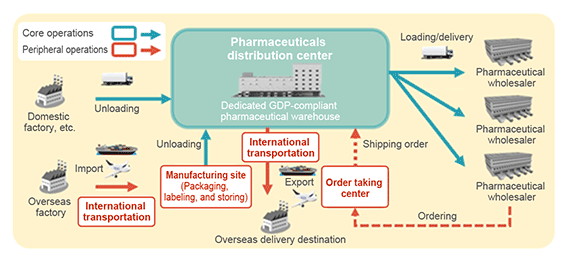
- Our core operations are the services from the unloading, acceptance and storage of supplies from manufacturing factories, manufacturing contractors, suppliers and other locations to the loading and delivery to distributors and other delivery destinations across Japan. Our peripheral operations include import/export, pharmaceuticals manufacturing operations and order management, all of which are used by many customers.
- We offer services that are Good Distribution Practice (GDP) compliant, e.g., building quality management systems, the creation of various types of standard operating procedures (SOPs), controlling temperature during storage and delivery, the implementation of security, and providing education. We propose optimal solutions to customers.
- We have warehouses for pharmaceuticals nationwide, especially in eastern and western Japan, and propose the bases that best suit each individual customer.
Our warehouses are quake-resistant, seismic isolation structures prepared for earthquakes or other disasters, including our implementation of measures such as the installation of emergency power generators and underground tanks filled with diesel fuel.
Packaging, labeling, and storage workflow
- List of Licenses and Notifications Under the Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices
- License for wholesale distribution of pharmaceuticals
- License for manufacturing pharmaceuticals (packaging, labeling, and storage)
- License for manufacturing quasi-pharmaceutical products (packaging, labeling, and storage)
- Registration of manufacturing of medical devices
- Registration of manufacturing of in-vitro diagnostics
- License for selling specially controlled medical devices
- Notifications of selling controlled medical devices
- License for manufacturing pharmaceuticals for animals (packaging, labeling, and storage)
- Registration of manufacturing medical devices for animals
- License for manufacturing cosmetics (packaging, labeling, and storage)
Related services
Inquiries
About logistics in Japan
Reception hours9:00-12:00 13:00-17:00
Daily, excluding weekends, national holidays, and end-of-year and New Year holidays (from December 30 to January 4)